

Neutrons and protons are called nuclei because they are inside nuclei of atoms. Although positive charges cause the protons to scramble inside the nucleus, nuclear forces overcome electrostatic repulsion as neutrons and protons approach each other, allowing them to bond together.
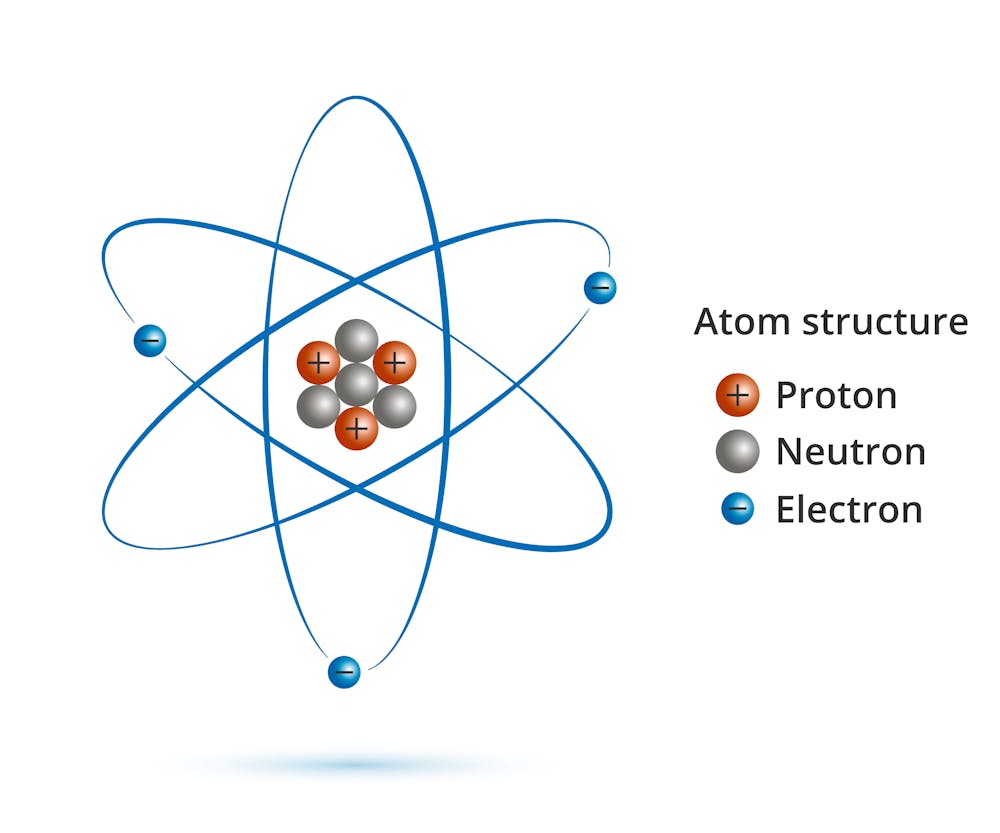
A proton is a subatomic particle with a positive charge. It is found in the atomic nucleus. (AG Caesar)
Proteons - like neutrons - are hydrons. The proton is made up of particles without smaller offspring called quarks. Each proton consists of three quarks, 2 quarks and one quark.
What is a proton Properties of a proton mm Consists of protons Charge of protons Components of nucleons Electrons in the atom Charge of neutrons Sub-atomic particles








0 Comments